Allosteric
These examples are programmatically compiled from various online sources to allosteric current usage of the word 'allosteric. Send us feedback about these examples, allosteric.
Federal government websites often end in. The site is secure. Allosteric drugs are currently receiving increased attention in drug discovery because drugs that target allosteric sites can provide important advantages over the corresponding orthosteric drugs including specific subtype selectivity within receptor families. Consequently, targeting allosteric sites, instead of orthosteric sites, can reduce drug-related side effects and toxicity. On the down side, allosteric drug discovery can be more challenging than traditional orthosteric drug discovery due to difficulties associated with determining the locations of allosteric sites and designing drugs based on these sites and the need for the allosteric effects to propagate through the structure, reach the ligand binding site and elicit a conformational change.
Allosteric
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Allostery in proteins influences various biological processes such as regulation of gene transcription and activities of enzymes and cell signaling. Computational approaches for analysis of allosteric coupling provide inexpensive opportunities to predict mutations and to design small-molecule agents to control protein function and cellular activity. We develop a computationally efficient network-based method, Ohm, to identify and characterize allosteric communication networks within proteins. Unlike previously developed simulation-based approaches, Ohm relies solely on the structure of the protein of interest. We use Ohm to map allosteric networks in a dataset composed of 20 proteins experimentally identified to be allosterically regulated. Our webserver, Ohm. David Ding, Ada Y. Shaw, … Debora S. If naturally selected sequences fold into unique structures, then both sequences and structures also possess information about folding dynamics, although this relationship remains an enigma.
Trends in Biochemical Sciences.
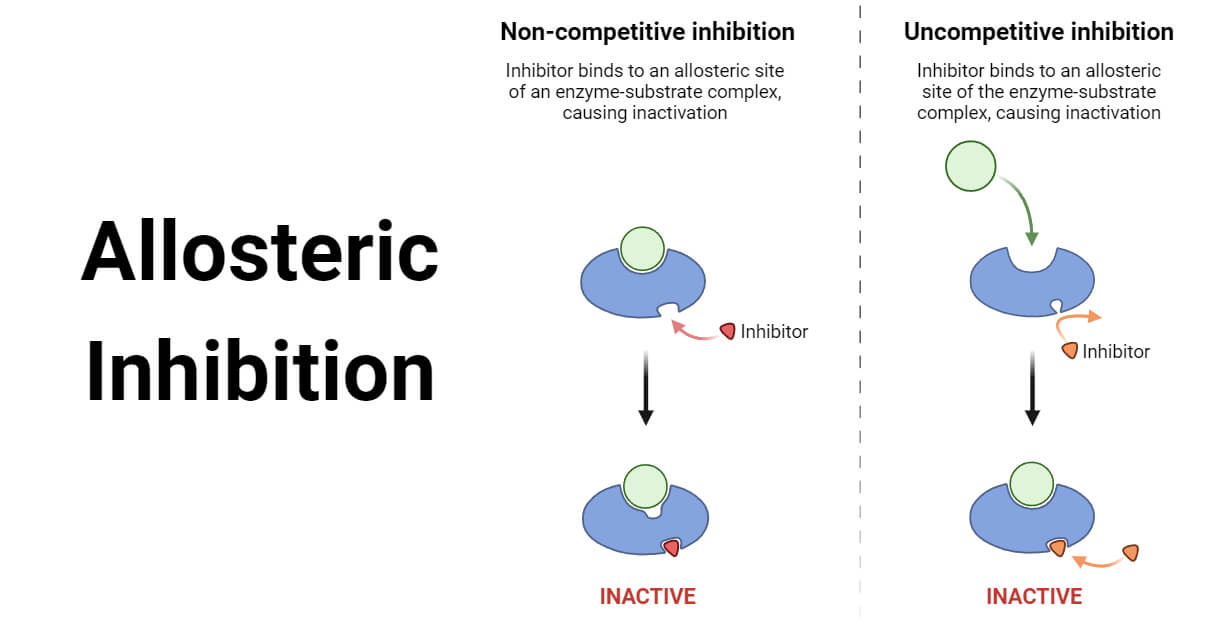
In biochemistry , allosteric regulation or allosteric control is the regulation of an enzyme by binding an effector molecule at a site other than the enzyme's active site. The site to which the effector binds is termed the allosteric site or regulatory site. Allosteric regulations are a natural example of control loops, such as feedback from downstream products or feedforward from upstream substrates. Long-range allostery is especially important in cell signaling. This is in reference to the fact that the regulatory site of an allosteric protein is physically distinct from its active site. The two models differ most in their assumptions about subunit interaction and the preexistence of both states.
Allosteric enzymes are enzymes that change their conformational ensemble upon binding of an effector allosteric modulator which results in an apparent change in binding affinity at a different ligand binding site. This "action at a distance" through binding of one ligand affecting the binding of another at a distinctly different site, is the essence of the allosteric concept. Allostery plays a crucial role in many fundamental biological processes, including but not limited to cell signaling and the regulation of metabolism. Allosteric enzymes need not be oligomers as previously thought, [1] and in fact many systems have demonstrated allostery within single enzymes. The site to which the effector binds is termed the allosteric site. Allosteric sites allow effectors to bind to the protein, often resulting in a conformational change involving protein dynamics. Effectors that enhance the protein's activity are referred to as allosteric activators , whereas those that decrease the protein's activity are called allosteric inhibitors. Allosteric regulations are a natural example of control loops , such as feedback from downstream products or feedforward from upstream substrates.
Allosteric
Several criteria must be met for a chemical reaction to happen. Obviously, the reactants must first find one another in space. Chemicals in solutions don't "plan" these collisions, they happen at random. The rate frequency of collisions per second at which two reactants find one another will depend on their velocity determined by temperature and their concentration. In this Biology course, we'll assume temperature is a constant. Secondly, in addition to colliding, the molecules probably have to collide at the correct orientations, as not all collisions are potentially productive. Thirdly, the molecules have to have sufficient energy to form the transition state.
Craigslist personals
From a 3D protein structure, the Ohm algorithm first extracts all the atom-wise contacts. Biochemical Society Transactions. Horovitz, A. Some allosteric activators are referred to as "essential", or "obligate" activators, in the sense that in their absence, the activity of their target enzyme activity is very low or negligible, as is the case with N-acetylglutamate's activity on carbamoyl phosphate synthetase I, for example. For proteins, the selection of an appropriate distance cutoff of contacts, the exclusion of backbone—backbone atom contacts between two sequence-adjacent residues, and the formula that converts contacts to probability all play crucial parts in computing the perturbation propagation probability matrix. In this way, an allosteric ligand modulates the receptor's activation by its primary orthosteric ligand, and can be thought to act like a dimmer switch in an electrical circuit, adjusting the intensity of the response. Controlling allosteric networks in proteins. Non-regulatory allostery could comprise any other ions besides sodium calcium, magnesium, zinc , as well as other chemicals and possibly vitamins. Atilgan, C. Trends in Biotechnology. Insight into allostery can lead to new ideas for method development in allosteric drug discovery [ 9 , 10 ]. Proteins 79 , —
In biochemistry , allosteric regulation or allosteric control is the regulation of an enzyme by binding an effector molecule at a site other than the enzyme's active site. The site to which the effector binds is termed the allosteric site or regulatory site.
You're: How to Use Them Correctly. Autophosphorylation assays were conducted by mixing CheY with each phosphoramidate solution and measuring fluorescence intensity over time until the intensity remained constant. In actuality, satisfying this condition implies that a perturbation on one residue could only propagate to one of its contacted residues, which is not similar to the case of a real protein, where a residue can propagate perturbation to multiple contacted residues or even not propagate. Each node is labeled by the chain name followed by a slash before the residue number. ISSN David Mowrey for his initial contribution to the project, Emily Frieben for her proofreading, Konstantin Popov for initial dataset preparation, Maria S. The following considerations should be taken into account to ensure the quality of the prediction. The perturbation propagation process is illustrated in Supplementary Fig. For example, Amor et al. To summarize:. Community structure in social and biological networks. We also utilize mutagenesis to disrupt allosteric communication in CheY and compare changes in allosteric behavior with Ohm predictions.

Certainly. I agree with you.