Al oh 3
Molar mass of Al OH 3 Aluminium hydroxide is Then, lookup atomic weights for each element in periodic table : Al:
The majority are aware that while aluminium oxide is an antacid, aluminium itself is a naturally occurring mineral. It treats stomach discomfort, acid indigestion, heartburn, and sour stomach. In addition, you may use it to lower phosphate levels in renal disease patients. Other non-medical uses for the antacid may be possible. It is the same as all existing other metal carbonates, sulphates, and hydroxides and is the chemical term for aluminium.
Al oh 3
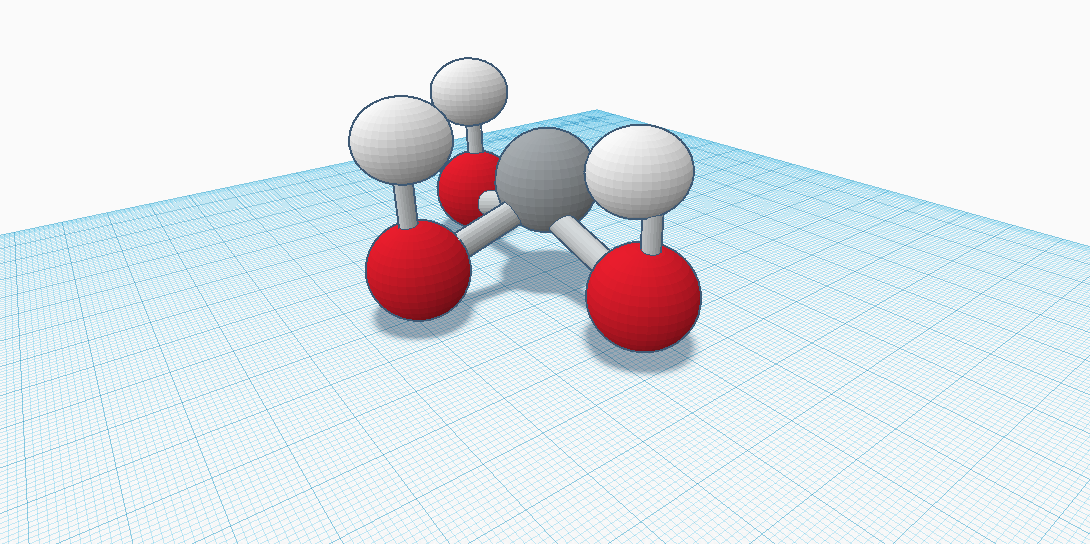
Aluminium hydroxide , Al OH 3 , is found in nature as the mineral gibbsite also known as hydrargillite and its three much rarer polymorphs : bayerite , doyleite , and nordstrandite. Aluminium hydroxide is amphoteric , i. Closely related are aluminium oxide hydroxide , AlO OH , and aluminium oxide or alumina Al 2 O 3 , the latter of which is also amphoteric. These compounds together are the major components of the aluminium ore bauxite. Aluminium hydroxide also forms a gelatinous precipitate in water. Al OH 3 is built up of double layers of hydroxyl groups with aluminium ions occupying two-thirds of the octahedral holes between the two layers. The polymorphs differ in terms of the stacking of the layers. All forms of Al OH 3 crystals are hexagonal [ disputed — discuss ] :. Hydrargillite , once thought to be aluminium hydroxide, is an aluminium phosphate. Nonetheless, both gibbsite and hydrargillite refer to the same polymorphism of aluminium hydroxide, with gibbsite used most commonly in the United States and hydrargillite used more often in Europe. Hydrargillite is named after the Greek words for water hydra and clay argylles. Aluminium hydroxide is amphoteric. It neutralizes the acid, yielding a salt: [9].
Wikimedia Commons.
It is found in nature in the form of mineral gibbsite and its polymorphs viz doyleite, nordstrandite, and bayerite. Aluminic hydroxide is an amorphous powder white. It is insoluble in water but soluble in alkaline and acidic solutions. Aluminic hydroxide has a typical structure of metal hydroxide consisting of hydrogen bonds. The reaction is as follows:.
Aluminum hydroxide is a common compound of aluminum, hydrogen, and oxygen that can be considered either a base, with the formula Al OH 3 , or an acid, with the formula H 3 AlO 3. The compound is frequently treated as a hydrate — a water-bonded compound — of aluminum oxide and designated variously as hydrated alumina, or aluminum hydrate or trihydrate, hydrated aluminum, or hydrated aluminum oxide, with the formula Al 2 O 3 H 2 0 x. Aluminum hydroxide is found in nature as the mineral bayerite or gibbsite also called hydrargillite. A mixed aluminum oxide-hydroxide mineral is known as diaspore or boehmite. In a purified form, aluminum hydroxide is either a bulky white powder or granules with a density of about 2. It is insoluble in water, but soluble in strong acids and bases.
Al oh 3
It is found in nature in the form of mineral gibbsite and its polymorphs viz doyleite, nordstrandite, and bayerite. Aluminic hydroxide is an amorphous powder white. It is insoluble in water but soluble in alkaline and acidic solutions. Aluminic hydroxide has a typical structure of metal hydroxide consisting of hydrogen bonds. The reaction is as follows:. It acts as a Lewis acid in bases. It takes away an electron pair from the hydroxide ions. The re4action is as follows:. Commercially used aluminium hydroxide is manufactured by the Bayer process. The waste is removed and the sodium aluminate solution is allowed to precipitate.
Houses for sale in holiday shores il
The dendritic cells pick up the antigen, carry it to lymph nodes , and stimulate T cells and B cells. The amorphous white powder is aluminium hydroxide. Dy OH 3. Th OH 4. While heating the solution, the color of this solution changes to white. Ac OH 3. Is aluminium hydroxide dangerous? One of the well-known brands of aluminium hydroxide adjuvant is Alhydrogel, made by Brenntag Biosector. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa. ISSN X. An additional negative impact of utilizing aluminium hydroxide:. Retrieved 2 July Side effects of aluminium hydroxide include intense stomach pain or constipation, lack of appetite; discomfort while urinating; muscle weakness, fatigue; extreme drowsiness.
Aluminium hydroxide , Al OH 3 , is found in nature as the mineral gibbsite also known as hydrargillite and its three much rarer polymorphs : bayerite , doyleite , and nordstrandite.
Weights of atoms and isotopes are from NIST article. Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets. Share Share Share Call Us. Tm OH 3. Hazard statements. Better still, get advice from a medical practitioner before ingesting any products that contain significant amounts of the mineral. Retrieved 3 July In patients with specific renal problems, aluminium oxide lowers phosphate levels. Retrieved 29 July Start Quiz. Retrieved 30 June It is biotic … What is Iodoform? View Result. Add them together: add the results from step 3 to get the total molar mass of the compound.

In my opinion you commit an error. I suggest it to discuss.
I apologise, but, in my opinion, you are not right. I am assured. I can defend the position.