Acidic nitrogen hydride
The correct answer is N 3 H.
A : H 2 O is the only hydride of chalcogen family which is liquid. R : Acidic nature of hydrides of chalcogen family increases down the group. Which of the following oxides of nitrogen is anhydride of nitric acid? A hydride of nitrogen which is acidic is. Hydride of nitrogen which is acidic is. A hydride of nitrogen which is acidic in nature is :.
Acidic nitrogen hydride
Hydrazoic acid , also known as hydrogen azide , azic acid or azoimide , [2] is a compound with the chemical formula HN 3. It is a compound of nitrogen and hydrogen , and is therefore a pnictogen hydride. Hydrazoic acid, like its fellow mineral acids , is soluble in water. The acid is usually formed by acidification of an azide salt like sodium azide. Normally solutions of sodium azide in water contain trace quantities of hydrazoic acid in equilibrium with the azide salt, but introduction of a stronger acid can convert the primary species in solution to hydrazoic acid. The pure acid may be subsequently obtained by fractional distillation as an extremely explosive colorless liquid with an unpleasant smell. Its aqueous solution can also be prepared by treatment of barium azide solution with dilute sulfuric acid , filtering the insoluble barium sulfate. It was originally prepared by the reaction of aqueous hydrazine with nitrous acid :. Other oxidizing agents, such as hydrogen peroxide , nitrosyl chloride , trichloramine or nitric acid , can also be used to produce hydrazoic acid from hydrazine. This reaction is unusual in that it involves compounds with nitrogen in four different oxidation states. In its properties hydrazoic acid shows some analogy to the halogen acids, since it forms poorly soluble in water lead, silver and mercury I salts. The metallic salts all crystallize in the anhydrous form and decompose on heating, leaving a residue of the pure metal. Azides of heavier alkali metals excluding lithium or alkaline earth metals are not explosive, but decompose in a more controlled way upon heating, releasing spectroscopically-pure N 2 gas. Hydrazoic acid may react with carbonyl derivatives, including aldehydes, ketones, and carboxylic acids, to give an amine or amide, with expulsion of nitrogen. This is called Schmidt reaction or Schmidt rearrangement.
Khimiya Geterotsiklicheskikh Soedinenii. Patna Civil Court Clerk. MP Cooperative Bank Clerk.
.
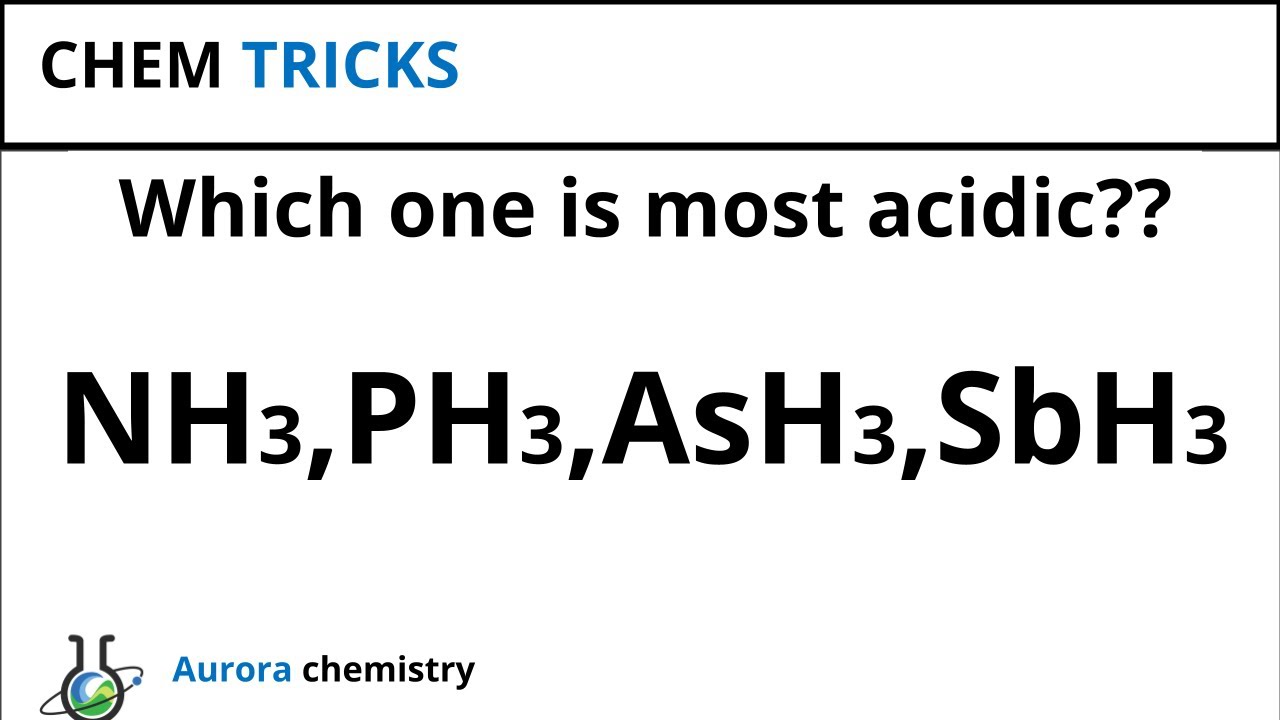
Post a Comment. Search This Blog. Tuesday, July 2, Hydrides of Nitrogen familyth Group:. AsH 3. SbH 3. BiH 3. Phosphine and other hydrides of heavier members of these groups are highly poisonous. E-H BL pm.
Acidic nitrogen hydride
With an accout for my. Although this ion does not exist except in extraordinary conditions, the term hydride is widely applied to describe compounds of hydrogen with other elements , particularly those of groups 1— The variety of compounds formed by hydrogen is vast, arguably greater than that of any other element. Every element of the periodic table except some noble gases forms one or more hydrides. These may be classified into three main types by the predominant nature of their bonding :. Aside from electride , the hydride ion is the simplest possible anion, consisting of two electrons and a proton.
Ticketmaster holland
Bihar Police Fireman. CISF Tradesman. Northern Coalfields Limited. Rajasthan Teacher. The compound acts as a non-cumulative poison. RRB Technician. Hydrazoic acid may react with carbonyl derivatives, including aldehydes, ketones, and carboxylic acids, to give an amine or amide, with expulsion of nitrogen. SSB Constable. BEL Senior Engineer. Assam Police Forester Grade I. Bihar Vidhan Sabha Junior Clerk. Oil India Senior Officer.
Metal-hydrogen bonds, also known misleadingly as metal hydrides , are ubiquitous X-type ligands in organometallic chemistry. They may be acidic or hydridic or both, depending on the nature of the metal center and the reaction conditions. Then again, the same can be said of X—H bonds in organic chemistry, which may vary from mildly nucleophilic consider Hantzsch esters and NADH to extremely electrophilic consider triflic acid.
HAL Management Trainee. Rajasthan CET. NVS Lab Attendant. Rajasthan Police Constable. Maharashtra Forest Department Stenographer. Bihar Police Fireman. Northern Coalfields Limited. Hydrocarbons alkanes alkenes alkynes Cycloalkanes Cycloalkenes Cycloalkynes Annulenes. DHS Assam Grade 4. In other projects. Acidic nitrogen hydride is DHS Assam Grade 3. Patna Civil Court Peon.

I consider, that you are mistaken. Let's discuss. Write to me in PM, we will communicate.
You have hit the mark. It seems to me it is excellent thought. I agree with you.
Tomorrow is a new day.