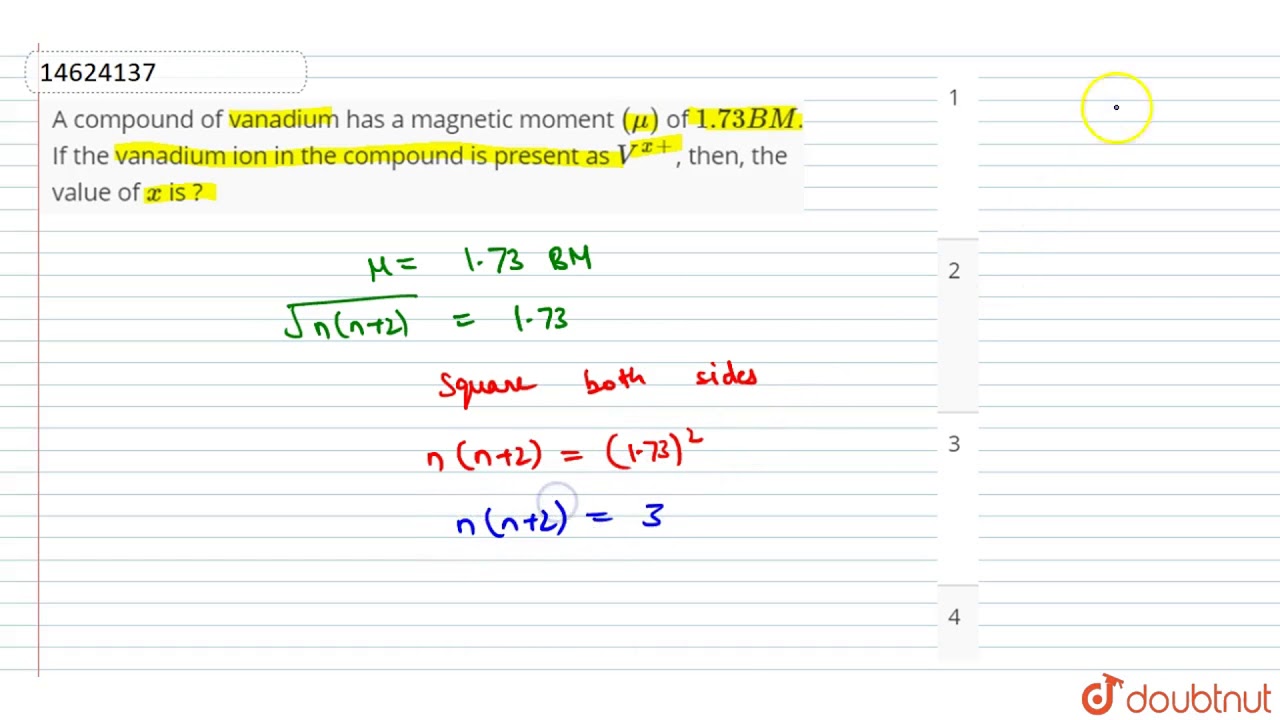
A compound of vanadium has a magnetic moment
Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. The magnetic moment of a transitiot metal of 3d series is 6.
Learn from their 1-to-1 discussion with Filo tutors. Total classes on Filo by this tutor - 10, Teaches : Physical Chemistry, Organic Chemistry. Total classes on Filo by this tutor - 7, Total classes on Filo by this tutor - 1, Teaches : Physics, Biology, Organic Chemistry. Views: 5,
A compound of vanadium has a magnetic moment
A compound of vanadium has a magnetic moment of 1. Work out the electronic configuration of vanadium ion in the compound. The electronic configuration of vanadium ion in the compound is:. What will be the electronic configurations:. Comprehension 1 Read the following rules and answer the questions at the end of it. Electrons in various suborbits of an filled in increasing order to their energies. Pairing of electrons in various orbitals of a suborbit takes places only after each orbital is half-filled. No two electrons in an atom can have the same set of quantum number. Electronic configuration of the vanadium ion in the compound is :. A compound of vanadium possesses a magnetic moment of 1. The oxidation state of vanadium in this compounds is:. The maximum magnetic moment is shown by the ion with electronic configuration.
Share Question Copy Link.
Doc 25 Pages. Sign in Open App. A compound of vanadium has a magnetic moment of 1. What is the electronic configuration of vanadium ion in the compound? Correct answer is option 'C'.
Submitted by Ashley S. Solved by verified expert. We will assign your question to a Numerade educator to answer. A compound of vanadium has a magnetic moment of 1. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. What is the chemical formula for the compound formed between vanadium III and bromine?
A compound of vanadium has a magnetic moment
A compound of vanadium has a magnetic moment of 1. The electronic configuration of vanadium ion in the compound is:. A compound of vanadium possesses a magnetic moment of 1. The oxidation state of vanadium in this compounds is:. What will be the electronic configurations:. A compound of vanadium chloride has spin only magnetic moment of 1. Its formula is. Chloro compound of Vanadium has only spin magnetic moment of 1.
Fran drescher nudes
Answer is not helpful. How many electron in an atom may have the following quantum number? Video Answer Solved by verified expert. Given the notation for the sub-shell deotected by the following quant Takes less than 10 seconds to signup. View Solution. Pairing of electrons in various orbitals of a suborbit takes places only after each orbital is half-filled. Snapsolve any problem by taking a picture. The Question and answers have been prepared according to the JEE exam syllabus. Show variable oxidation state Impart colour to flame Are paramagnetic in nature Act as catalytic agents. A compound of vanadium possesses a magnetic moment of 1. Problem
A compound of vanadium has a magnetic moment of 1. Work out the electronic configuration of vanadium ion in the compound. The electronic configuration of vanadium ion in the compound is:.
Give two9 point of difference between The compound can also be prepared using XeF6 as the starting compound. What is the lowest value of n that allows g orbitals to exist? To be left with only one unpaired electron, vanadium atom should lose four electrons i. Arihant Sanubia Salim, Yukta Khatri. A compound of vanadium chloride has spin only magnetic moment of 1. This gives us the electronic configuration [Ar] 4s0 3d1. High dosage tutoring from Dedicated 3 experts. The angular momentum vector associated with an atomic state can take up only certain specified directions in space. Select a course to view your unattempted tests. More from Related Course. In the experiment, silver is vapourized in an electric oven and silver atoms spray into the evacuated apparatus through a small hole in the oven wall. Was this solution helpful? Continue with Google.

0 thoughts on “A compound of vanadium has a magnetic moment”