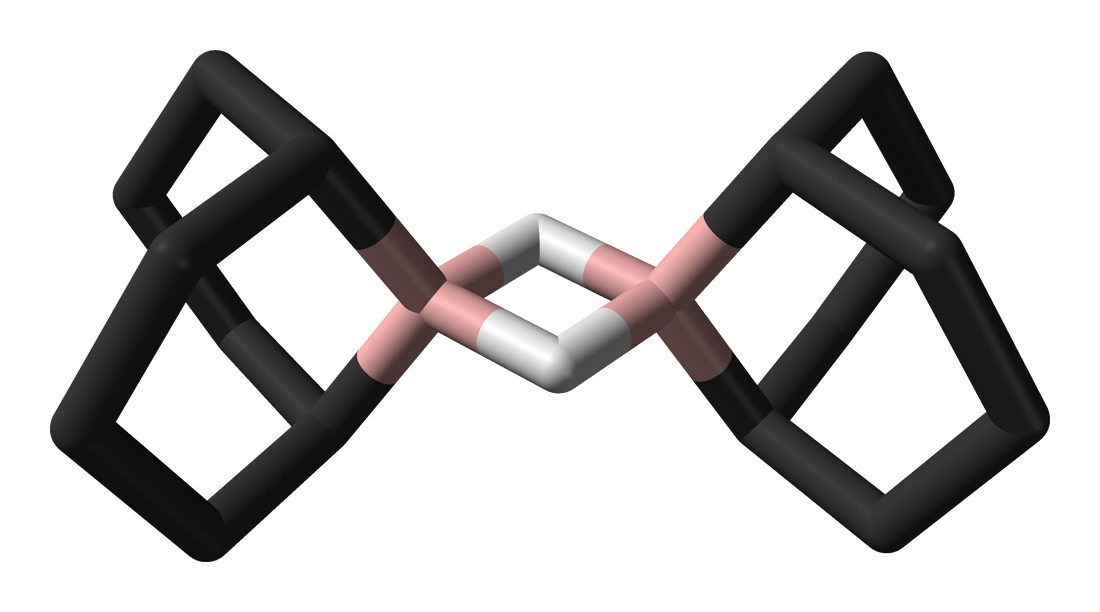
9 bbn structure
From Wikimedia Commons, the free media repository.
This colourless solid is used in organic chemistry as a hydroboration reagent. The compound exists as a hydride-bridged dimer, which easily cleaves in the presence of reducible substrates. The compound is commercially available as a solution in tetrahydrofuran and as a solid. Its highly regioselective addition on alkenes allows the preparation of terminal alcohols by subsequent oxidative cleavage with H 2 O 2 in aqueous KOH. The steric demand of 9-BBN greatly suppresses the formation of the 2-substituted isomer compared to the use of borane. Contents move to sidebar hide. Article Talk.
9 bbn structure
.
PMC
.
An especially valuable group of intermediates can be prepared by addition of an compound to carbon-carbon double or triple bonds:. The reaction is called hydroboration and is a versatile synthesis of organoboron compounds. These additions amount to reduction of both carbons of the double bond:. Organoboranes can be considered to be organometallic compounds. Elemental boron does not have the properties of a metal, and boron-carbon bonds are more covalent than ionic. Hydroboration and the many uses of organoboranes in synthesis were developed largely by H. Brown and co-workers. In our discussion, we shall give more detail on hydroboration itself, and then describe several useful transformations of organoboranes. The reactions usually are carried out in ether solvents, although hydrocarbon solvents can be used with the borane-dimethyl sulfide complex.
9 bbn structure
The hydroboration-oxidation of alkynes is similar to the reaction with alkenes. However, there is one important difference. The alkyne has two pi bonds and both are capable of reacting with borane BH 3. To limit the reactivity to only one of the pi bonds within the alkyne, a dialkyl borane reagent R 2 BH is used.
Hairstyles using headbands
Scientific American Blog Network. Infobox references. The source code of this SVG is invalid due to 13 errors. The substitution of any brackets is due to technical restrictions. This image of a simple structural formula is ineligible for copyright and therefore in the public domain , because it consists entirely of information that is common property and contains no original authorship. PubChem CID. Su-no-G talk contribs. Interactive image. You cannot overwrite this file. GHS labelling :. In other projects. Deutsch: Strukturformel von monomerem 9-Borabicyclo[3. CAS Number. ISBN Organic Syntheses via Boranes.
B -alkylBBN preparation is itself a two-step process, thus restricting the utility of the 9-BBN for synthetic purposes. These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
EC Number. Toggle limited content width. Category : 9-BBN. In other projects. The compound is commercially available as a solution in tetrahydrofuran and as a solid. H , H , H Chemical compound. Captions Captions English Add a one-line explanation of what this file represents. File information. SVG development InfoField. Main page Welcome Community portal Village pump Help center.

This very valuable opinion