2agcl
Silver chloride is an inorganic chemical compound with the chemical formula Ag Cl, 2agcl.
Then, lookup atomic weights for each element in periodic table : Ba: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'.
2agcl
.
Crystal structure. FeCl 2 FeCl 3.
.
Silver chloride is an inorganic chemical compound with the chemical formula Ag Cl. This white crystalline solid is well known for its low solubility in water and its sensitivity to light. Upon illumination or heating, silver chloride converts to silver and chlorine , which is signaled by grey to black or purplish coloration in some samples. AgCl occurs naturally as the mineral chlorargyrite. It is produced by a metathesis reaction for use in photography and in pH meters as electrodes. Silver chloride is unusual in that, unlike most chloride salts, it has very low solubility.
2agcl
Silver chloride is a white crystalline chemical compound with the formula AgCl. Silver chloride in the test tube quickly turns purplish, especially in a sunny laboratory because the silver chloride is split up into silver and chlorine. Silver chloride is prepared when sodium chloride is added to silver nitrate solution a white precipitate of silver chloride occurs. Silver chloride is an example of a well-known salt stain used to impart an amber colour to the glass.
Easy pencil shading for kids
Chemical forum. Hull; D. Download as PDF Printable version. Find atomic masses: look up the atomic masses of each element present in the compound. PbCl 2 PbCl 4. SgO 2 Cl 2. Other names Cerargyrite Chlorargyrite Horn silver Argentous chloride. The solubility product , K sp , for AgCl in water is 1. In other projects. FeCl 2 FeCl 3. Unit converters. Silver-based photographic films were first made in by Johann Heinrich Schulze with silver nitrate. Bibcode : Mate S2CID Example: calculating molar mass Let's calculate the molar mass of carbon dioxide CO 2 : Carbon C has an atomic mass of about
Well you gots the stoichiometric equation And silver chloride is as soluble as a BRICK, and precipitates from aqueous solution as a curdy white solid. When you try to collect the silver chloride it is a very curdy, unhandy precipitate.
Greenwood; A. The silver chloride that forms will precipitate immediately. In , Schulze made a paste of silver nitrate and chalk, placed the mixture in a glass bottle, and wrapped the bottle in Other anions. Download as PDF Printable version. Silver chloride and silver nitrate have been used in photography since it began, and are well known for their light sensitivity. Contact us. Arno Press. Sechkarev Chemistry of the Elements 2 ed. TmCl 2 TmCl 3. Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. Of these reactions used to leach silver chloride from silver ores, cyanidation is the most commonly used.

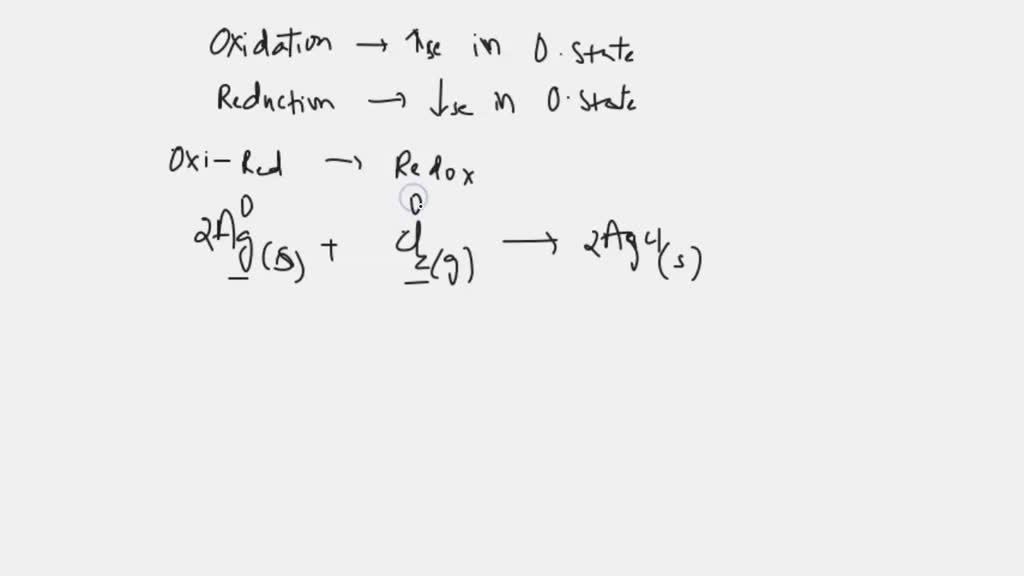
I think, that you are mistaken. I suggest it to discuss. Write to me in PM, we will talk.
You are absolutely right. In it something is also idea excellent, I support.
Many thanks for an explanation, now I will know.